Multiple Choice
Tetracyanoethylene has the skeleton shown below: 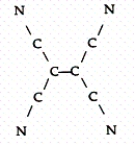 From its Lewis structure determine the following:
From its Lewis structure determine the following:
-How many nonbonded electron pairs are in the molecule?
A) 0
B) 2
C) 4
D) 5
E) 8
Correct Answer:

Verified
Correct Answer:
Verified
Q26: Which of the following are paramagnetic?<br>O<sub>2 </sub>O<sub>2</sub><sup>-</sup><sup>
Q27: In the molecule C<sub>2</sub>H<sub>4</sub> the valence orbitals
Q28: Which of the following statements is incorrect?<br>A)For
Q29: Which of the following species has the
Q30: Consider the molecule and the following hybridization
Q32: The hybridization of Cl in ClF<sub>2</sub><sup>+</sup> is<br>A)sp<br>B)sp<sup>2</sup><br>C)sp<sup>3</sup><br>D)dsp<sup>3</sup><br>E)d<sup>2</sup>sp<sup>3</sup>
Q33: Which of the following species is paramagnetic?<br>A)C<sub>2</sub><br>B)O<sub>2</sub><br>C)F<sub>2</sub><br>D)Li<sub>2</sub><br>E)none
Q34: What is the bond order of Ne<sub>2</sub>?<br>A)0<br>B)
Q35: A <span class="ql-formula" data-value="\pi"><span class="katex"><span
Q36: The H<sub>2</sub><sup>-</sup> ion is more stable than