Multiple Choice
Tetracyanoethylene has the skeleton shown below: 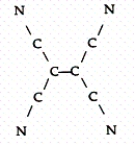 From its Lewis structure determine the following:
From its Lewis structure determine the following:
-How many of the atoms are sp hybridized?
A) 2
B) 4
C) 6
D) 8
E) 10
Correct Answer:

Verified
Correct Answer:
Verified
Q40: The hybridization of Se in SeF<sub>6</sub> is<br>A)sp<br>B)sp<sup>2</sup><br>C)sp<sup>3</sup><br>D)dsp<sup>3</sup><br>E)d<sup>2</sup>sp<sup>3</sup>
Q41: If a molecule demonstrates paramagnetism,then :<br>I.The substance
Q42: The hybridization of the central atom in
Q43: Which of the nitrogen-containing molecules below is
Q44: Which of the following has the largest
Q46: Explain the concept of delocalization of electrons
Q47: Which of the following statements about the
Q48: According to MO theory,F<sub>2</sub> should be diamagnetic.
Q49: For how many of the following does
Q50: Draw a molecular orbital diagram for O<sub>2</sub>