Multiple Choice
Complete the Lewis structure for the following molecule: 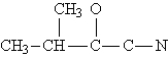 This molecule has __________ sigma and __________ pi bonds.
This molecule has __________ sigma and __________ pi bonds.
A) 4,5
B) 6,3
C) 11,5
D) 13,2
E) 13,3
Correct Answer:

Verified
Correct Answer:
Verified
Q87: When comparing Be<sub>2</sub> and H<sub>2</sub>:<br>I.Be<sub>2</sub> is
Q88: Consider the molecule and the following hybridization
Q89: Consider the following Lewis structure: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q90: Consider the benzene molecule.Which of the following
Q91: Consider the molecule and the following hybridization
Q93: Which of the following statements about the
Q94: Which of the following molecules or ions
Q95: Atoms that are sp<sup>2</sup> hybridized form _
Q96: The hybridization of the B in BH<sub>3</sub>
Q97: The electron configuration of a particular