Multiple Choice
Use the molecules below to answer the next three questions. 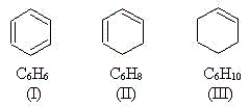
-Which molecule(s) have p orbitals that share an electron pair to create bonding?
A) I
B) II
C) III
D) all of the above
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q74: Which of the following molecules contains a
Q75: Consider the molecule C<sub>2</sub>H<sub>4</sub>.The hybridization of each
Q76: Which of the following diatomic molecules has
Q77: The fact that O<sub>2</sub> is paramagnetic can
Q78: What hybridization is predicted for the nitrogen
Q80: The bond order in the NO molecule
Q81: Which of the following has the greatest
Q82: The following statements concern molecules that require
Q83: The CO molecule has the bond order:<br>A)0<br>B)1<br>C)2<br>D)3<br>E)4
Q84: The hybridization of Br in BrF<sub>3</sub> is<br>A)sp<br>B)sp<sup>2</sup><br>C)sp<sup>3</sup><br>D)dsp<sup>3</sup><br>E)d<sup>2</sup>sp<sup>3</sup>