Multiple Choice
Given the following information: Br2 bond energy = 193 kJ/mol
F2 bond energy = 154 kJ/mol 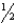 Br2(g) +
Br2(g) + 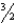 F2(g) BrF3(g)
F2(g) BrF3(g)
H° = -384 kJ/mol
Calculate the Br-F bond energy.
A) 244 kJ/mol
B) 237 kJ/mol
C) 712 kJ/mol
D) 128 kJ/mol
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q41: When a metal reacts with a nonmetal
Q42: In the reaction between magnesium and sulfur,the
Q43: How many of the following molecules or
Q44: Draw the Lewis structures of the molecules
Q45: As indicated by Lewis structures,which of the
Q47: When several nonequivalent Lewis structures can be
Q48: Which of the following pairs is isoelectronic?<br>A)Li<sup>+</sup>
Q49: Calculate the lattice energy for LiCl(s)given the
Q50: Choose the molecule with the strongest bond.<br>A)HF<br>B)HCl<br>C)HBr<br>D)HI<br>E)All
Q51: In which of the following compounds does