Multiple Choice
When molten sulfur reacts with chlorine gas,a vile-smelling orange liquid forms that is found to have the empirical formula SCl.Which of the following could be the correct Lewis structure for this compound?
A) 
B) 
C) 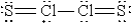
D) 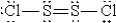
E) 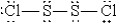 :
:
Correct Answer:

Verified
Correct Answer:
Verified
Q71: The ability of an atom in a
Q72: In the Lewis structure for ICl<sub>2</sub><sup>-</sup>,how many
Q73: Consider the compound crotonaldehyde,whose skeleton is: <img
Q74: Which of the following molecules has a
Q75: Which of the following molecules exhibits the
Q77: A molecule that has a center of
Q78: Choose the molecule with the strongest bond.<br>A)F<sub>2</sub><br>B)Cl<sub>2</sub><br>C)Br<sub>2</sub><br>D)I<sub>2</sub><br>E)All
Q79: Choose the statement that best describes the
Q80: What is the correct order of the
Q81: Which of these is an isoelectronic series?<br>A)Na<sup>+</sup>,K<sup>+</sup>,Rb<sup>+</sup>,Cs<sup>+</sup><br>B)K<sup>+</sup>,Ca<sup>2+</sup>,Ar,S<sup>2</sup><sup>-</sup><br>C)Na<sup>+</sup>,Mg<sup>2+</sup>,S<sup>2</sup><sup>-</sup>,Cl<sup>-</sup><br>D)Li,Be,B,C<br>E)none