Multiple Choice
Given the following Lewis structure: 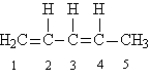
-How many unshared pairs of electrons are present in this molecule?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: The _ is the change in energy
Q19: The Cl-Kr-Cl bond angle in KrCl<sub>4</sub> is
Q20: Which of the following statements is false?<br>A)Models
Q21: Which of the following ionic compounds has
Q22: Which of the following molecules contains a
Q24: Which of the following species is best
Q25: Consider the following molecules.<br>I.BF<sub>3</sub><br>II.CHBr<sub>3</sub> (C is the
Q26: For the elements Cs,F,and P,the order of
Q27: For each of the following compounds:<br>a)Draw the
Q28: Which of the following is not a