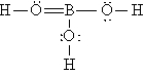
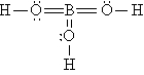
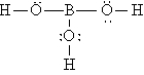
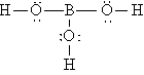
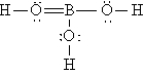
Q92: A nonpolar covalent bond results from the
Q93: Which has the greater N-O bond length,NO<sub>2</sub><sup>-</sup>
Q94: The bond angle in H<sub>2</sub>Se is about:<br>A)120°<br>B)60°<br>C)180°<br>D)109°<br>E)90°
Q95: Choose the molecule with the strongest bond.<br>A)CH<sub>4</sub><br>B)H<sub>2</sub>O<br>C)NH<sub>3</sub><br>D)HF<br>E)All
Q96: In the molecule XeF<sub>2</sub>,how many pairs of
Q98: Which ion is planar?<br>A)NH<sub>4</sub><sup>+</sup><br>B)CO<sub>3</sub><sup>2</sup><sup>-</sup><br>C)SO<sub>3</sub><sup>2</sup><sup>-</sup><br>D)ClO<sub>3</sub><sup>-</sup><br>E)all are planar
Q99: For each of the following compounds:<br>a)Draw the
Q100: Which of the following has an incomplete
Q101: The Lewis structure for CHCl<sub>3</sub> has nine
Q102: Consider the compound crotonaldehyde,whose skeleton is: <img