Multiple Choice
Which of the following Lewis structures best describes BF3?
A) 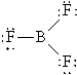
B) 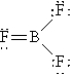
C) 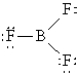
D) 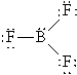
E) 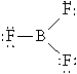
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: Select the molecule from the following that
Q86: The size in a series of isoelectronic
Q87: How many of the following molecules possess
Q88: Match the ions below with the pictures
Q89: The shape of an ammonia molecule is
Q91: Of the following,which molecule has the largest
Q92: A nonpolar covalent bond results from the
Q93: Which has the greater N-O bond length,NO<sub>2</sub><sup>-</sup>
Q94: The bond angle in H<sub>2</sub>Se is about:<br>A)120°<br>B)60°<br>C)180°<br>D)109°<br>E)90°
Q95: Choose the molecule with the strongest bond.<br>A)CH<sub>4</sub><br>B)H<sub>2</sub>O<br>C)NH<sub>3</sub><br>D)HF<br>E)All