Multiple Choice
Consider the following representation of a 2p-orbital: 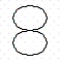 Which of the following statements best describes the movement of electrons in a p-orbital?
Which of the following statements best describes the movement of electrons in a p-orbital?
A) The electrons move along the outer surface of the p-orbital,similar to a "figure 8" type of movement.
B) The electrons move within the two lobes of the p-orbital,but never beyond the outside surface of the orbital.
C) The electrons are concentrated at the center (node) of the two lobes.
D) The electrons are only moving in one lobe at any given time.
E) The electron movement cannot be exactly determined.
Correct Answer:

Verified
Correct Answer:
Verified
Q123: From the following list of observations,choose the
Q124: The statement that "the lowest energy configuration
Q125: How many electrons in an atom can
Q126: A line in the spectrum of
Q127: Fe has _ that is (are)unpaired in
Q129: List the following atoms in order of
Q130: Which of the following is a reasonable
Q131: Write the electron configuration for the following:<br>-P
Q132: The _ quantum number is related to
Q133: Of the following elements,which needs three electrons