Multiple Choice
Which of the following combinations of quantum numbers is not allowed?
A) n = 1,l = 1,ml = 0,ms = 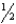
B) n = 3,l = 0,ml = 0,ms = - 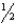
C) n = 2,l = 1,ml = -1,ms = 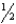
D) n = 4,l = 3,ml = -2,ms = - 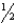
E) n = 4,l = 2,ml = 0,ms = 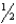
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: If l = 3,how many electrons can
Q26: The number of unpaired electrons in the
Q27: Which of the following atoms has the
Q28: A point in the wave function where
Q29: Consider the ionization energy (IE)of the magnesium
Q31: An element E has the electron configuration
Q32: The _ electrons are in the outermost
Q33: How many electrons in an atom can
Q34: An element with the electron configuration [Xe]
Q35: Which of the following statements is true