Essay
Consider the graph below to answer the next two questions: 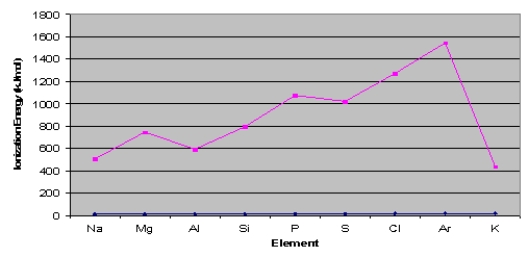
-Explain why argon has the highest ionization energy.
Correct Answer:

Verified
Argon has the highest ionization energy ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Argon has the highest ionization energy ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q90: If n = 2,how many orbitals are
Q91: The number of orbitals having a given
Q92: An element has the electron configuration [Kr]
Q93: Which of the following are incorrectly
Q94: Write the electron configuration for the following:<br>-S<sup>2</sup><sup>-</sup>
Q96: The small,but important,energy differences between 3s,3p,and 3d
Q97: For the set of elements Li,O,Ne,and Na,which
Q98: What is the l quantum number for
Q99: The SI unit for frequency is cycles
Q100: Choose the atom or ion using a