Essay
Write balanced equations for each of the processes,choosing from the following substances as reactants: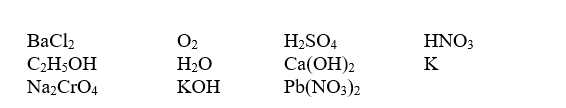
-Precipitation of BaSO4 from solution
Correct Answer:

Verified
H2SO...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q73: Which of the following ions is most
Q74: The oxidation state of iodine in IO<sub>3</sub><sup>-</sup>
Q75: How many of the following are
Q76: Selecting from the following reagents,indicate which reagents
Q77: Which of the following reactions does
Q79: You have exposed electrodes of a light
Q80: The net ionic equation contains which of
Q81: Phosphoric acid,H<sub>3</sub>PO<sub>4</sub>,is a triprotic acid.What is the
Q82: Which of the following are oxidation-reduction
Q83: Which of the following statements is not