Multiple Choice
Consider the fermentation reaction of glucose: 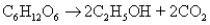 A 1.00-mole sample of C6H12O6 was placed in a vat with 100 g of yeast.If 32.3 grams of C2H5OH was obtained,what was the percent yield of C2H5OH?
A 1.00-mole sample of C6H12O6 was placed in a vat with 100 g of yeast.If 32.3 grams of C2H5OH was obtained,what was the percent yield of C2H5OH?
A) 35.1%
B) 17.5%
C) 100%
D) 32.3%
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q69: Suppose the reaction Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> + 3H<sub>2</sub>SO<sub>4</sub>
Q70: A 0.4987-g sample of a compound known
Q71: One molecule of a compound weighs
Q72: Nitric oxide,NO,is made from the oxidation
Q73: The atomic mass of rhenium is 186.2.Given
Q75: Which of the following equations correctly
Q76: A hydrocarbon (a compound consisting solely of
Q77: Which of the following statements is
Q78: You have a sample of zinc (Zn)and
Q79: What is the coefficient for oxygen