Multiple Choice
The following equation describes the oxidation of ethanol to acetic acid by potassium permanganate: 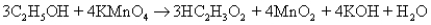 5.00 g of ethanol and an excess of aqueous KMnO4 are reacted,and 4.24 g of HC2H3O2 result.What is the percent yield?
5.00 g of ethanol and an excess of aqueous KMnO4 are reacted,and 4.24 g of HC2H3O2 result.What is the percent yield?
A) 100%
B) 65.1%
C) 21.7%
D) 34.9%
E) 4.24 g HC2H3O2 is impossible since it represents more than 100% yield.
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Calculate the molar mass of barium sulfite.<br>A)233.40
Q36: A substance contains 23.0 g sodium,27.0 g
Q37: The limiting reactant in a reaction<br>A)has the
Q38: Iron is biologically important in the
Q39: Sulfuric acid may be produced by
Q41: A 7.11-g sample of potassium chlorate
Q42: The reactant with the highest molar mass
Q43: Potassium forms an oxide containing 1 oxygen
Q44: The empirical formula of styrene is CH;its
Q45: The empirical formula of a group of