Multiple Choice
Here are some crystal field representations of d electrons in an octahedral complex:
I) 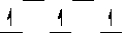 II)
II)  III)
III) 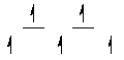 IV)
IV) 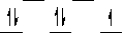 V)
V) 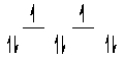 Choose the representation that fits the transition metal atom in the following species:
Choose the representation that fits the transition metal atom in the following species:
-K4Mn(CN) 6 (assume strong field)
A) representation I
B) representation II
C) representation III
D) representation IV
E) representation V
Correct Answer:

Verified
Correct Answer:
Verified
Q65: The complex FeL<sub>6</sub><sup>2+</sup>,where L is a neutral
Q66: All tetrahedral complex ions are high spin.
Q67: What are the oxidation numbers of each
Q68: The complex ions containing Zn<sup>2+</sup> are intensely
Q69: Which of the following ligands are capable
Q71: How many d electrons are present on
Q72: Addition of AgNO<sub>3</sub> to aqueous solutions
Q73: Which of the following is true?<br>A)The first
Q74: The electron configuration of Ti<sup>2+</sup> is<br>A)[Ar] 4s<sup>2</sup><br>B)[Ar]
Q75: The spectrochemical series is I<sup>-</sup> < Br<sup>-</sup>