Multiple Choice
Here are some crystal field representations of d electrons in an octahedral complex:
I) 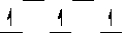 II)
II)  III)
III) 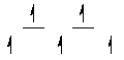 IV)
IV) 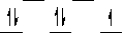 V)
V) 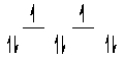 Choose the representation that fits the transition metal atom in the following species:
Choose the representation that fits the transition metal atom in the following species:
-K4Fe(CN) 6
A) representation I
B) representation II
C) representation III
D) representation IV
E) representation V
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Name the careful heat treatment of metals
Q9: Which of the following complexes would be
Q10: A complex ion is a charged species
Q11: Copper(I)complexes would be expected to be colorless.
Q12: Why do transition metals show a lot
Q14: How many unpaired electrons are there in
Q15: Which model(s)accounts for the magnetism and color
Q16: True or false: Transition metals show great
Q17: How many unpaired electrons are found in
Q18: Which of the following statements concerning