Multiple Choice
A complex ion is a square planar complex.It has a d8 electron configuration.What is the most reasonable d orbital scheme for this complex?
A) 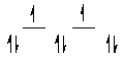
B) 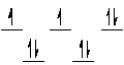
C) 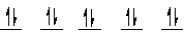
D) 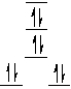
E) 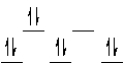
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q108: Because they have the same atoms,bonds,and formulas,geometrical
Q109: Analysis of the data from a titration
Q110: Carboxyhemoglobin is formed when _ prevents the
Q111: Which metal ion has a d<sup>5</sup> electron
Q112: Which complex ion shape is not capable
Q114: According to crystal field theory,how many unpaired
Q115: What is the sum of all isomers
Q116: How many unpaired electrons are found in
Q117: Here are some crystal field representations of
Q118: Which of the following transition metals is