Multiple Choice
Which of the following crystal field diagrams is correct for Mn(CN) 63- (CN- is a strong field ligand) ?
A) 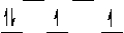
B) 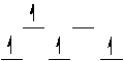
C) 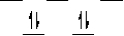
D) 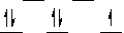
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q88: Calculate the total number of unpaired electrons
Q89: How many unpaired electrons are there in
Q90: The complex ions of Zn<sup>2+</sup> are all
Q91: The electron configuration for Cr<sup>2+</sup> is<br>A)[Ar] 4s<sup>2</sup>3d<sup>4</sup><br>B)[Ar]
Q92: What transition metal has the combination of
Q94: Which of the following crystal field diagrams
Q95: How many unpaired electrons are there in
Q96: Which of the following statements is true
Q97: How many unpaired electrons are found in
Q98: The complex ion NiCl<sub>4</sub><sup>2</sup><sup>-</sup> is tetrahedral.The number