Multiple Choice
A certain complex ion has a distorted octahedral structure in which the ligands along the plus and minus z axes are compressed (pushed in closer to the central metal ion) .The d orbital splitting diagram for this complex ion would be:
A) ________ 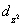 ________
________ 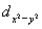
________ ________ 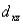
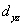
________ 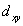
B) ________ ________ 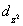
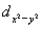 ________ ________ ________
________ ________ ________ 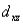
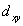
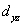
C) ________ 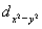 ________
________ 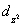
________ 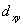
________ ________ 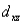
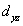
D) ________ ________ ________ 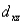
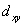
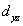 ________ ________
________ ________ 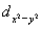
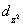
E) ________ ________ 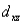
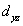 ________
________ 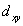
________ 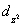
________ 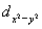
Correct Answer:

Verified
Correct Answer:
Verified
Q71: How many d electrons are present on
Q72: Addition of AgNO<sub>3</sub> to aqueous solutions
Q73: Which of the following is true?<br>A)The first
Q74: The electron configuration of Ti<sup>2+</sup> is<br>A)[Ar] 4s<sup>2</sup><br>B)[Ar]
Q75: The spectrochemical series is I<sup>-</sup> < Br<sup>-</sup>
Q77: The transition metal _ is present in
Q78: What is the formula for the pentaamminehydroxocopper(II)ion?<br>A)[Cu(NH<sub>3</sub>)(OH)<sub>5</sub>]<sup>3</sup><sup>-</sup><br>B)[Cu(NH<sub>3</sub>)<sub>5</sub>(OH)<sub>5</sub>]<sup>2+</sup><br>C)[Cu(NH<sub>3</sub>)<sub>5</sub>(OH)]<sup>2+</sup><br>D)[Cu(NH<sub>3</sub>)<sub>5</sub>(OH)<sub>5</sub>]<sup>3</sup><sup>-</sup><br>E)[Cu(NH<sub>3</sub>)<sub>5</sub>(OH)]<sup>+</sup>
Q79: The complex ion Ni(NH<sub>3</sub>)<sub>6</sub><sup>2+</sup> (two unpaired electrons)is
Q80: The reducing abilities of the first-row transition
Q81: The phenomenon called _ contraction is responsible