Essay
Consider the pseudo-octahedral complex of Cr3+ shown below,where A and B represent Lewis bases and where A produces a stronger crystal field than B.Draw an appropriate crystal field diagram for this complex (include the electrons). 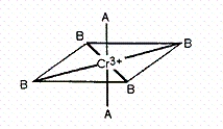
Correct Answer:

Verified
________  ________
________  ________
________
________ 
 ...
...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
________
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q94: Which of the following crystal field diagrams
Q95: How many unpaired electrons are there in
Q96: Which of the following statements is true
Q97: How many unpaired electrons are found in
Q98: The complex ion NiCl<sub>4</sub><sup>2</sup><sup>-</sup> is tetrahedral.The number
Q100: NiCl<sub>4</sub><sup>2</sup><sup>-</sup> (tetrahedral)<br>A)0<br>B)1<br>C)2<br>D)4<br>E)5
Q101: The iron in hemoglobin is _ when
Q102: The expected electron configuration of Cu<sup>+</sup> is
Q103: The complex ion Co(NH<sub>3</sub>)<sub>6</sub><sup>2+</sup> (three unpaired electrons)is
Q104: Which 3d transition metal is mixed with