Multiple Choice
The major industrial source of hydrogen gas is the reaction of methane and water at high temperatures (800 - 1000°C) and high pressures (10 - 15 atm) with nickel as a catalyst. CH4(g) + H2O(g) 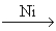 CO2(g) + 3H2(g)
CO2(g) + 3H2(g)
If 171.5 g of CH4 and 171.5 g of H2O are reacted at 945°C and 11.0 atm,how much hydrogen should be available for industrial use?
A) 201 L
B) 291 L
C) 259 L
D) 28.8 L
E) 86.5 L
Correct Answer:

Verified
Correct Answer:
Verified
Q48: Choose the correct molecular structure for NH<sub>4</sub><sup>+</sup>.<br>A)trigonal
Q49: Which of the following is not a
Q50: Which of the following oxides is amphoteric?<br>A)BeO<br>B)MgO<br>C)CaO<br>D)SrO<br>E)BaO
Q51: Choose the group containing the most reactive
Q52: Within a group,as the atomic numbers of
Q54: The solid substance with the empirical formula
Q55: Which of the following ions interferes with
Q56: What element is found in the structural
Q57: Write a balanced equation for each of
Q58: Why is nitrogen not able to form