Multiple Choice
Identify the missing particle in the following nuclear equation: 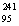 Am +
Am + 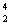 He __________ + 2
He __________ + 2 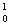 n
n
A) 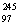 Bk
Bk
B) 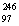 Bk
Bk
C) 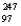 Bk
Bk
D) 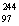 Bk
Bk
E) 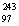 Bk
Bk
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The Br-82 nucleus has a half-life
Q2: Consider a certain type of nucleus that
Q3: What nuclide is necessary to balance
Q4: Iron-56 ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt=" Iron-56
Q6: An unstable isotope of rhenium,<sup>191</sup>Re,has a half-life
Q7: An archaeological sample contains 0.739 g
Q8: The nuclide <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt=" The
Q9: Which of the following statements (A-D)is false?<br>A)The
Q10: It is desired to determine the
Q11: Consider a certain type of nucleus