Multiple Choice
One of the hopes for solving the world's energy problem is to make use of the fusion reaction: 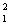 H +
H + 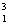 H
H 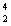 He +
He + 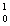 n + energy How much energy is released when one mole of deuterium is fused with one mole of tritium according to the above reaction? The masses of the atoms and the neutrons are:
n + energy How much energy is released when one mole of deuterium is fused with one mole of tritium according to the above reaction? The masses of the atoms and the neutrons are: 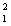 H = 2.0140 amu
H = 2.0140 amu 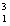 H = 3.01605 amu
H = 3.01605 amu 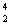 He = 4.002603 amu
He = 4.002603 amu 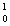 n = 1.008665 amu
n = 1.008665 amu
The speed of light is 2.9979 108 m/s
A) 5.63 108 J
B) 56.3 J
C) 1.69 1012 J
D) 7.84 1044 J
E) 8.44 1011 J
Correct Answer:

Verified
Correct Answer:
Verified
Q64: Breeder reactors are used to convert the
Q65: The bismuth-209 nucleus has a binding
Q66: The Fe-56 nucleus is known to
Q67: Consider a certain type of nucleus that
Q68: The half-life of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="The half-life
Q70: The smallest amount of radioactive material that
Q71: A radioactive element has a half-life of
Q72: Identify the missing particle in the
Q73: The I-131 nuclide has a half-life of
Q74: Electron capture transforms <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Electron capture