Multiple Choice
Calculate the change in energy in kJ/mol for the transmutation of radium from the given molar masses: 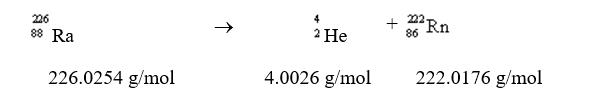
A) -5.2 kJ/mol
B) -1.6 kJ/mol
C) -4.7 1014 kJ/mol
D) -4.7 108 kJ/mol
E) +1.6 108 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q73: The I-131 nuclide has a half-life of
Q74: Electron capture transforms <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Electron capture
Q75: The lead-208 nucleus has a mass
Q76: A certain radioactive sample contains 2.4
Q77: The most likely decay mode (or
Q79: Use the following data to determine the
Q80: The number of a certain radioactive nuclide
Q81: The nuclide <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt=" The
Q82: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q83: If a tree dies and the trunk