Multiple Choice
The concentration of OH- in a saturated solution of Mg(OH) 2 is 3.63 10-4 M.The Ksp of Mg(OH) 2 is
A) 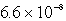
B) 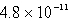
C) 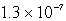
D) 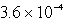
E) 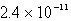
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q73: A 0.012-mol sample of Na<sub>2</sub>SO<sub>4</sub> is
Q74: It is observed that 7.50 mmol of
Q75: Will precipitation occur?<br>A)Yes.<br>B)No.<br>C)Maybe,it depends on the temperature.<br>D)Maybe,it
Q76: In the qualitative analysis scheme for metal
Q77: What is the maximum concentration of
Q79: A solution is 0.010 M in each
Q80: Consider a solution made by mixing
Q81: The K<sub>sp</sub> of AgI is 1.5
Q82: The K<sub>sp</sub> of Al(OH)<sub>3</sub> is 2
Q83: The solubility of Mg(OH)<sub>2</sub> (K<sub>sp</sub> =