Multiple Choice
The solubility of silver phosphate,Ag3PO4, at 25°C is 1.60 10-5 mol/L.What is the Ksp for the silver phosphate at 25°C?
A) 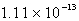
B) 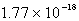
C) 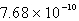
D) 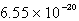
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The best explanation for the dissolution of
Q5: The K<sub>sp</sub> for Mn(OH)<sub>2</sub> is 2.0
Q6: The following questions refer to the
Q7: Determine the equilibrium concentration of the
Q8: The solubility of La(IO<sub>3</sub>)<sub>3</sub> in a 0.42
Q10: Calculate the solubility of Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> (K<sub>sp</sub>
Q11: An unknown salt,M<sub>2</sub>Z,has a K<sub>sp</sub> of <img
Q12: Consider a solution containing the following cations:
Q13: Calculate the solubility of Ag<sub>2</sub>CrO<sub>4</sub> (K<sub>sp</sub>
Q14: An industrial plant processes its waste