Multiple Choice
A 300.0-mL saturated solution of copper(II) peroidate,Cu(IO4) 2,contains 0.30 grams of dissolved salt.Determine the Ksp.
A) 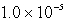
B) 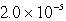
C) 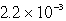
D) 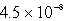
E) 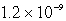
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Barium carbonate has a measured solubility
Q22: Which of the following compounds has
Q23: Find the solubility (in mol/L)of lead(II)chloride,PbCl<sub>2</sub>,at
Q24: The solubility in mol/L of M(OH)<sub>2</sub>
Q25: What is the concentration of Ni<sup>2+</sup>(aq)ion
Q27: Calculate the concentration of the silver
Q28: The solubility in mol/L of Ag<sub>2</sub>CrO<sub>4</sub>
Q29: The K<sub>f</sub> for the complex ion
Q30: In the classic scheme for qualitative analysis,the
Q31: Which of the following solid salts is