Multiple Choice
Calculate the solubility of Cu(OH) 2 in a solution buffered at pH = 7.59.(Ksp = 1.6 10-19)
A) 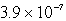 M
M
B) 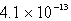 M
M
C) 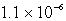 M
M
D) 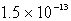 M
M
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q66: The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0
Q67: What is the best way to ensure
Q68: How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub>
Q69: If 30 mL of 5.0
Q70: The following questions refer to the
Q72: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt=" The
Q73: A 0.012-mol sample of Na<sub>2</sub>SO<sub>4</sub> is
Q74: It is observed that 7.50 mmol of
Q75: Will precipitation occur?<br>A)Yes.<br>B)No.<br>C)Maybe,it depends on the temperature.<br>D)Maybe,it
Q76: In the qualitative analysis scheme for metal