Consider the Following Information About the Diprotic Acid,ascorbic Acid 10-5)
HAs- As2- + H+ pKa
Multiple Choice
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As  HAs- + H+ pKa
HAs- + H+ pKa
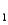 = 4.10 (Ka
= 4.10 (Ka
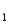 = 7.9 10-5)
= 7.9 10-5)
HAs-  As2- + H+ pKa
As2- + H+ pKa
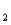 = 11.79 (Ka
= 11.79 (Ka
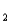 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below: 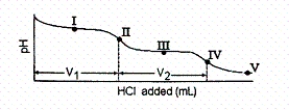
-What is the pH at point I (V1/2 HCl added) ?
A) 4.10
B) 7.95
C) 11.79
D) 12.39
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q80: A 100.mL sample of 0.10 M
Q81: Consider a solution consisting of the following
Q82: If 25.0 mL of 0.451 M NaOH
Q83: A 19.0-mL sample of tartaric acid is
Q84: Consider the titration of 100.0 mL of
Q86: The pH at the equivalence point of
Q87: Given 100.0 mL of a buffer
Q88: You are given 5.00 mL of an
Q89: Which of the following will not produce
Q90: How many moles of solid NaF