Multiple Choice
A solution containing 10.mmol of 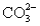 and 5.0 mmol of
and 5.0 mmol of 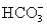 is titrated with 1.8 M HCl.What volume of HCl must be added to reach the first equivalence point?
is titrated with 1.8 M HCl.What volume of HCl must be added to reach the first equivalence point?
A) 2.8 mL
B) 5.6 mL
C) 10.6 mL
D) 15.6 mL
E) 20.6 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q59: Consider the titration of 100.0 mL of
Q60: For a solution equimolar in HCN and
Q61: Consider the titration of 100.0 mL of
Q62: Which of the following is the
Q63: What volume of 0.0100 M NaOH
Q65: What is the percent dissociation of
Q66: Consider the titration of 300.0 mL
Q67: Consider the titration of 100.0 mL
Q68: Consider a solution of 2.0 M
Q69: A solution of hydrochloric acid of unknown