Multiple Choice
The equilibrium constant for the reaction A- + H+  HA is called:
HA is called:
A) Ka
B) Kb
C) 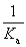
D) 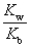
E) KwKa
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: The hydrogen halides (HF,HCl,HBr,and HI)are all polar
Q5: What is the equilibrium concentration of
Q6: Calculate the pH of a 0.49
Q7: As water is heated,its pH decreases.This means
Q8: The pK<sub>a</sub> of HOCl is 7.5.Calculate the
Q10: Given that the K<sub>a</sub> for HOCl
Q11: The two acid dissociation constants for
Q12: The pH of a 0.118 M
Q13: Consider the reaction HNO<sub>2</sub>(aq)+ H<sub>2</sub>O(l) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q14: Calculate the pOH of a 4.9 M