Multiple Choice
Given the following acids and Ka values: 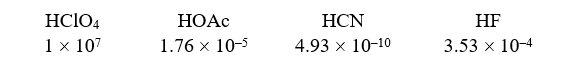
What is the order of increasing base strength?
A) CN-,F-,OAc-,ClO4-
B) CN-,OAc-,F-,ClO4-
C) CN-,ClO4-,F-,OAc-
D) ClO4-,OAc-,CN-,F-
E) ClO4-,F-,OAc-,CN-
Correct Answer:

Verified
Correct Answer:
Verified
Q23: At 65°C,the ion-product constant of water,K<sub>w</sub>,is
Q24: Determine whether the following oxides produce an
Q25: A solution of 8.01 M formic
Q26: Select the answer that best describes an
Q27: The [H<sub>3</sub>O<sup>+</sup>] of a 0.49 M
Q29: Calculate the pH of the following aqueous
Q30: Carbonic acid is a diprotic acid,H<sub>2</sub>CO<sub>3</sub>,with
Q31: Explain why Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub> produces an acidic solution
Q32: Calculate the pH of the following aqueous
Q33: Calculate the [H<sup>+</sup>] in a 0.068