Multiple Choice
The equilibrium constant for the reaction NH4+ + OH-  NH3 + H2O is:
NH3 + H2O is:
A) 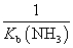
B) 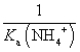
C) 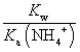
D) 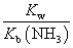
E) 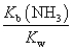
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: Which of the following is not
Q59: Calculate the pH of a 2.28 M
Q60: Calculate the percentage of pyridine (C<sub>5</sub>H<sub>5</sub>N)that
Q61: Calculate the pOH of a 0.12
Q62: Calculate the pH of a 0.13
Q64: The following question refers to a
Q65: Calculate the pH of the following aqueous
Q66: What is the equilibrium concentration of
Q67: Consider a 0.70 M solution of HOCl.If
Q68: Hypobromous acid,HOBr,has an acid dissociation constant