Multiple Choice
If Ka for HCN is 6.17 10-10,what is Kb for CN-? Note: CN- + H2O  HCN + OH-
HCN + OH- 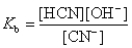
A) 6.17 10-24
B) 6.17 104
C) 1.62 10-5
D) 1.23 10-9
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q72: A 0.33-mol sample of a diprotic
Q73: Which is the strongest acid of the
Q74: Saccharin is a monoprotic acid.If the
Q75: Which of the following solutions contains
Q76: The following three equations represent equilibria that
Q78: The pH of a 0.17 M
Q79: Calculate the pH of a 0.47
Q80: Calculate the [H<sup>+</sup>] in a solution
Q81: The pain killer morphine is a
Q82: Acetic acid, (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)is a weak acid