Multiple Choice
The OH· radical disproportionates according to the elementary chemical reaction 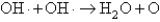 This reaction is second order in OH·.The rate constant for the reaction is 2.0 10-12 cm3/molecules at room temperature.If the initial OH concentration is 1.3 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second order in OH·.The rate constant for the reaction is 2.0 10-12 cm3/molecules at room temperature.If the initial OH concentration is 1.3 1013 molecules/cm3,what is the first half-life for the reaction?
A) 3.5 1011 s
B) 2.6 101 s
C) 3.8 10-2 s
D) 7.7 10-14 s
E) 1.9 10-2 s
Correct Answer:

Verified
Correct Answer:
Verified
Q79: The rate constant k for the reaction
Q80: The following questions refer to the
Q81: Which of the following statements best describes
Q82: The rate constant for a reaction at
Q83: The following questions refer to the
Q85: The reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="The reaction
Q86: The concentration of O<sub>2</sub> at t
Q87: The balanced equation for the reaction of
Q88: Under certain conditions the reaction H<sub>2</sub>O<sub>2</sub>
Q89: In 6 M HCl,the complex ion Ru(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup>