The Decomposition of N2O5(g)to NO2(g)and O2(g)obeys First-Order Kinetics 10-5 S-1 at 25°C,what Is the Half-Life for the Reaction
Multiple Choice
The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics.Assuming the form of the rate law is: 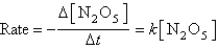
Where k = 5.4 10-5 s-1 at 25°C,what is the half-life for the reaction described?
A) 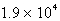 s
s
B) 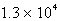 s
s
C) 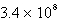 s
s
D) 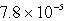 s
s
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q69: The elementary chemical reaction O +
Q70: The rate constant k is dependent on<br>I.the
Q71: Consider the reaction 3A + B
Q72: The reaction H<sub>2</sub>SeO<sub>3</sub>(aq)6I<sup>-</sup>(aq)+ 4H<sup>+</sup>(aq) <span class="ql-formula"
Q73: If the reaction were reversible,would the forward
Q75: Use the potential energy diagram shown to
Q76: The kinetics of the reaction <img
Q77: The reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt=" The
Q78: The reaction 3NO <span class="ql-formula"
Q79: The rate constant k for the reaction