Multiple Choice
According to collision theory,the activated complex that forms in step 1 could have which of the following structures? (The dotted lines represent partial bonds. )
A) 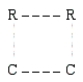
B) 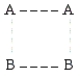
C) 
D) 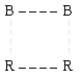
E) 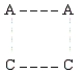
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Consider the following data concerning the
Q20: The experimental rate law for the
Q21: The kinetics of the reaction <img
Q22: Consider the reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Consider the
Q23: Which of the following statements about enzymes
Q25: The following initial rate data were
Q26: A general reaction written as A
Q27: For the reaction aA ? Products, use
Q28: The reaction A <span class="ql-formula"
Q29: The half-life of this reaction is approximately<br>A)15