Multiple Choice
Use the following drawing of a gaseous solute in equilibrium with a solution to help answer the question below. 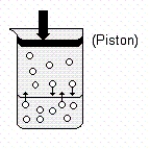 Which of the following statements are true when the piston is pushed in (downward) ?
Which of the following statements are true when the piston is pushed in (downward) ?
A) This will cause the pressure of the gas to increase and the concentration of the dissolved gas to go down.
B) This will cause the pressure of the gas to decrease and the concentration of the dissolved gas to go down.
C) This will cause the pressure of the gas to increase and the concentration of the dissolved gas to go up.
D) This will cause the volume of the gas to decrease and the concentration of the dissolved gas to go down.
E) This will cause the volume of the gas to increase and the concentration of the dissolved gas to go up.
Correct Answer:

Verified
Correct Answer:
Verified
Q98: A chemist is given a white
Q99: A solution of CH<sub>3</sub>OH in H<sub>2</sub>O would
Q100: We can predict the solubility of a
Q101: How many molecules of sucrose (table
Q102: The osmotic pressure of a 0.0100 M
Q103: Find the mass percent of CuSO<sub>4</sub> in
Q104: In osmosis:<br>A)Knowing the osmotic pressure can help
Q105: For each of the following solutions,describe the
Q106: The vapor pressure of water at 25.0°C
Q108: Which of the following chemical or physical