Multiple Choice
Aluminum metal crystallizes in a face-centered cubic structure.The relationship between the radius of an Al atom (r) and the length of an edge of the unit cell (E) is:
A) r = E/2
B) 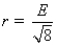
C) 
D) r = 2E
E) 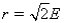
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q73: Chromium metal crystallizes as a body-centered cubic
Q74: Solid MgO has the same crystal structure
Q75: Lithium chloride crystallizes in a face-centered
Q76: In a(n)_ alloy some of the host
Q77: Which of the following should have the
Q79: Which of the following statements is (are)false?<br>I.The
Q80: Atomic solids generally have low melting points.
Q81: Which of the following processes must exist
Q82: Steel is a substitutional alloy.
Q83: Which of the following has the highest