Multiple Choice
Based on the phase diagram shown below,which of the following statements are correct? 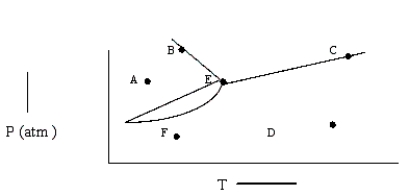
I.Sublimation occurs at a point in the transformation that occurs along a straight line from point A to point F.
II.C and E represent points where the gas and liquid phases are in equilibrium.
III. Hvap can be measured at point B.
IV.Molecules at point D have a greater average kinetic energy than those at point F.
V.The temperature at point E is called the critical temperature of the compound.
A) II,V
B) I,III,IV
C) I,II,III
D) II,IV,V
E) I,II,IV
Correct Answer:

Verified
Correct Answer:
Verified
Q81: Which of the following processes must exist
Q82: Steel is a substitutional alloy.
Q83: Which of the following has the highest
Q84: A certain solid substance that is very
Q85: At normal atmospheric pressure and a temperature
Q87: The volume of a single cell.<br>A)1.20
Q88: Which of these statements is false?<br>A)Diamond is
Q89: Methane (CH<sub>4</sub>)exhibits stronger hydrogen bond interactions than
Q90: Knowing that <span class="ql-formula" data-value="\Delta"><span
Q91: Which of the following compounds has the