Multiple Choice
A monolayer containing 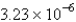 g of oleic acid has an area of 20.0 cm2.The density of oleic acid is 0.895 g / mL.What is the thickness of the monolayer (the length of an oleic acid molecule) ?
g of oleic acid has an area of 20.0 cm2.The density of oleic acid is 0.895 g / mL.What is the thickness of the monolayer (the length of an oleic acid molecule) ?
A) 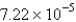 cm
cm
B) 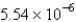 cm
cm
C) 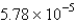 cm
cm
D) 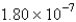 cm
cm
E) 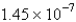 cm
cm
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Explain the main differences between a compound
Q14: The calibration points for the linear Reaumur
Q15: Suppose that you purchased a water
Q16: What are the components of the scientific
Q17: Using the rules of significant figures,calculate the
Q19: Color changes always indicate a chemical change.
Q20: An example of a pure substance is<br>A)elements<br>B)compounds<br>C)pure
Q21: In 1928,29.3 g of a new element
Q22: In the spring of 2008,petrol cost £1.179
Q23: In 1984,some drums of uranium hexafluoride were