Multiple Choice
The following questions are based on the reaction A + B ↔ C + D shown in Figure 6.4.
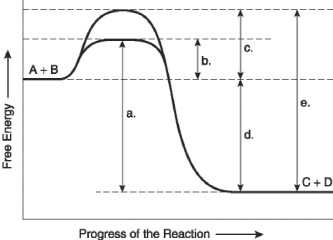 Figure 6.4
Figure 6.4
-Which of the following terms best describes the forward reaction in Figure 6.4?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
E) chemical equilibrium, ∆G = 0
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The following questions are from the end-of-chapter
Q13: For the hydrolysis of ATP to ADP
Q25: When ATP releases some energy, it also
Q33: Whenever energy is transformed, there is always
Q36: Reactants capable of interacting to form products
Q36: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 6.1 -Which
Q38: The following question(s) are based on the
Q50: For living organisms, which of the following
Q76: Which of the following is an example
Q78: Which of the following statements is true