Multiple Choice
Which one of the following pairs of atoms would be most likely to form ions and thus an ionic bond?
A) 
B) 
C) 
D) 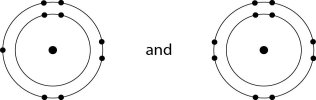
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Why is each element unique and different
Q12: Which of the following molecules contains the
Q13: Which of the following statements is false?<br>A)
Q15: The molar mass of glucose (C6H12O6) is
Q16: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6147/.jpg" alt=" Figure 2.1 -Which
Q18: One idea to mitigate the effects of
Q56: What is the difference between covalent bonds
Q61: From its atomic number of 15, it
Q114: Phosphorus-32, a radioactive isotope of phosphorus-31 (atomic
Q128: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.4 -How