Multiple Choice
Figure 2.9
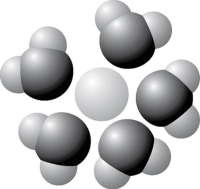
-Based on your knowledge of the polarity of water molecules, the solute molecule depicted in Figure 2.9 is most likely
A) positively charged.
B) negatively charged.
C) without charge.
D) hydrophobic.
E) nonpolar.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: An atom has 6 electrons in its
Q21: We can be sure that a mole
Q22: Buffers are substances that help resist shifts
Q29: Why does ice float in liquid water?<br>A)
Q72: Molybdenum has an atomic number of 42.
Q86: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt=" Figure 2.4 -In
Q97: Which bond or interaction would be difficult
Q100: Carbon dioxide (CO2) is readily soluble in
Q103: Assume that acid rain has lowered the
Q107: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.3 -Which