Multiple Choice
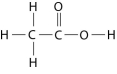 Figure 2.10
Figure 2.10
-How many grams would be equal to 1 mol of the compound shown in Figure 2.10? (carbon = 12, oxygen = 16, hydrogen = 1)
A) 29
B) 30
C) 60
D) 150
E) 342
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: The slight negative charge at one end
Q14: The atomic number of nitrogen is 7.
Q38: What is the maximum number of covalent
Q64: Liquid water's high specific heat is mainly
Q65: Many mammals control their body temperature by
Q71: One mole (mol) of glucose (molecular mass
Q72: The nucleus of a nitrogen atom contains
Q76: The reactivity of an atom arises from<br>A)
Q78: Based on electron configuration, which of these
Q83: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.6 -What