Multiple Choice
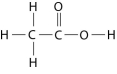 Figure 2.10
Figure 2.10
-How many grams of the compound in Figure 2.10 would be required to make 1 L of a 0.5 M solution? (carbon = 12, oxygen = 16, hydrogen = 1)
A) 29
B) 30
C) 60
D) 150
E) 342
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: The molar mass of glucose (C6H12O6) is
Q16: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6147/.jpg" alt=" Figure 2.1 -Which
Q18: One idea to mitigate the effects of
Q21: A group of molecular biologists is trying
Q22: Measurements show that the pH of a
Q25: Which of the following would be regarded
Q52: An atom with atomic number 12 would
Q56: What is the pH of a solution
Q61: From its atomic number of 15, it
Q114: Phosphorus-32, a radioactive isotope of phosphorus-31 (atomic