Multiple Choice
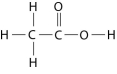 Figure 2.10
Figure 2.10
-How many grams of the compound in Figure 2.10 would be required to make 2.5 L of a 1 M solution? (carbon = 12, oxygen = 16, hydrogen = 1)
A) 29
B) 30
C) 60
D) 150
E) 342
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Which of the following statements is true
Q38: What is the maximum number of covalent
Q51: A dietary Calorie equals 1 kilocalorie. Which
Q55: One liter of a solution of pH
Q64: Liquid water's high specific heat is mainly
Q67: If the pH of a solution is
Q76: The reactivity of an atom arises from<br>A)
Q76: What is the maximum number of electrons
Q78: Based on electron configuration, which of these
Q79: What coefficients must be placed in the