Multiple Choice
Identical heat lamps are arranged to shine on identical containers of water and methanol (wood alcohol) , so that each liquid absorbs the same amount of energy minute by minute. The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
A) 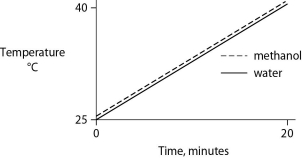
B) 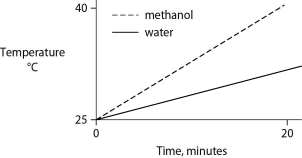
C) 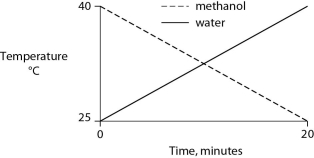
D) 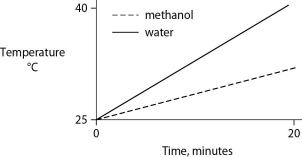
E) 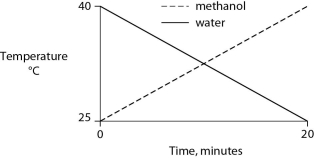
Correct Answer:

Verified
Correct Answer:
Verified
Q1: You have a freshly prepared 0.1 M
Q2: What results from an unequal sharing of
Q3: Carbon dioxide (CO2) is readily soluble in
Q7: The partial negative charge in a molecule
Q11: Why is each element unique and different
Q14: Consider two solutions: solution X has a
Q23: In ammonium chloride salt (NH4Cl), the anion
Q56: What is the difference between covalent bonds
Q63: Fluorine has an atomic number of 9
Q128: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.4 -How