Multiple Choice
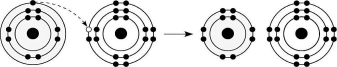 Figure 2.6
Figure 2.6
-Which of the atoms shown would be most likely to form an anion with a charge of -1?
A) 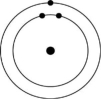
B) 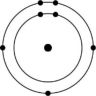
C) 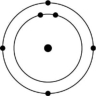
D) 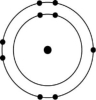
E) 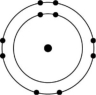
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: How would acidification of seawater affect marine
Q13: The slight negative charge at one end
Q56: What is the difference between covalent bonds
Q113: You have two beakers.One contains pure water;the
Q118: The nucleus of a nitrogen atom contains
Q120: The atomic number of sulfur is 16.
Q124: Sulfur is in the same column of
Q125: If acid rain has lowered the pH
Q126: What is the hydroxyl ion (OH-) concentration
Q127: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.3 -Which